Time-resolved fluorescence spectroscopic studies
From SMOSwiki
Introduction
Time-resolved fluorescence spectroscopy is one of the widely used research tools in science contaning chemistry, biology and physics [1]. As fluorescence decay lifetime of a dye molecule is very sensitive to its local environment, it gives information about the microscopic environment of the dye molecule. Our research is focused on understanding the relaxation dynamics and photophysical and photochemical properties of guest-host systems such as the dyes incorporated in zeolite matrices and dye molecules in various liquid media.
Photophysical properties of dye molecules in zeolites
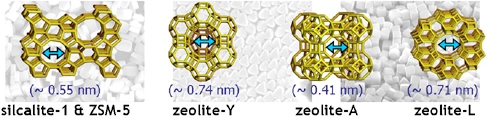
Zeolite is nanoporous materials which consist of inorganic matter. The environment of zeolite is very uniform and well-defined. Especially, zeolite having narrow pores can be well isolated from the ohter moleucles. However, fundamental questions are still being asked about how the incoporated molecules react, organize, and interact each other in inside of the zeolite. We use Steady-state and time-resolved spectroscopy (TCSPC) technique to investigate the photophysical and photochemical properties of incoporated dye molecules in different zeolites.
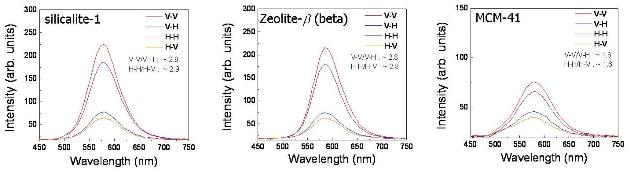
We prepared hemicyanine dye molecules incoporated in nanoporous materials (silicalite-1, zeolite-β, MCM-41) with different pore sizes. The pore diameters were 0.55 nm, 0.73 nm, and 3 ~ 6 nm for silicalite-1, zeolite-β, and MCM-41, respectively. From the measured fluorescence anisotropy and time-resolved fluorescence results, the dye molecuels in zeolite were found to be clearly well-aligned, along the straight channel of the zeolite, except for the case of MCM-41 having lager pores and we could evaluated that the dye molecules incorporated in small straight channels of silicalite-1 are in very homogenous environment.
Photophysical properties of ionic liquid
In most materials peak of emission spectrum does not change with the change in excitation wavelength. It is called as Kasha's rule and arised from the fast relaxation from higher electronic and vibrational levels (see Fig.1)[2]. However, peak wavelength of emission from pure ionic liquid was observed to shift over 100 nm following the change in the excitation wavelength[3].
At first, we study 4-[4-(dimethylamino)styryl]-1-n-alkylpyridinium bromide (hemicyanine) dissolved in 1-butyl-3-methylimidazolium tetrafluoroborate, [BMIM][BF4], and 1-butyl-3-methylimidazolium hexafluorophosphate, [BMIM][PF6]). The fluorescence from hemicyanine in [BMIM][BF4] and [BMIM][PF6] red-shifted continuously throughout the whole range of the absorption spectrum with the increase in the excitation wavelength. This phenomenon has been known for other viscous matrices such as low-temperature glasses, highly viscous liquid, or in polymer matrices as named red-edge effect[4]. However, fluorescence ionic liquids shift much larger than quantity expected that the relaxation time law in red-edge effect.

So, scientists guess that anormalous fluorescene is contributed by other reasons. Firstly, impurities affect on fluorescence spectra of RILs[6]. Secondly, molecular structures (Dimer, Trimer, any other aggregates) in ionic liquids are other candidate of specical fluorescence properties of ionic liquids. Finally, Dynamic Heterogeneity is most popular assumption that explaining fluorescence shift in ionic liquids[7][8][9][10][11].
Now, we are studying fluorescence properteis of ionic liquids by fluorescence correlation spectroscopy (FCS) to check for the possibility of the existence of molecular aggregates of varying sizes as an origin of the observed fluorescence, fluorescence correlation spectroscopy was employed to measure the size of fluorescent species in the ionic liquid-water at mixtures, prepared at different concentrations to hinder the possible aggregate formation in a changeable manner.
Dye molecules in ionic liquids
References:
- ↑ (2006).
- ↑ J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer. (2006)
- ↑ A. Samanta, J. Phys. Chem. B 110, 13704 (2006)
- ↑ A. P. Demchenko, Luminescence 7, 19 (2002)
- ↑ Shim et al., J. Phys. Chem B 112, 1906 (2008)
- ↑ A. Paul, P. K. Mandal, A. Samanta, Chem. Phys. Lett. 402, 375 (2005)
- ↑ P. K. Mandal, A. Paul, A. Samanta, J. Photochem. Photobiol. 182, 113 (2006)
- ↑ H. Jin et al, J. Phys. Chem B 111, 7291 (2007)
- ↑ H. Jin and M. Maroncelli, J. Phys. Chem B 111, 13473 (2007)
- ↑ Z. Hu and C. J. Margulis, Proc. Natl. Acad. Sci. USA 103, 831 (2006)
- ↑ Z. Hu and C. J. Margulis, Acc. Chem. Res 40, 1097 (2007)