Ionic liquid
From SMOSwiki
The Room-temperature Ionic Liquid (IL) is a salt consisting of certain organic cation and anion, and is in liquid phase at room temperature. Even though ILs have been known for ~100 years, ILs are gaining much interest only recently (from 1999 by Welton [1]). It is because many ILs are nonvolatile and inflammable, yet can dissolve many different kinds of chemicals. The possbile applications of ionic liquids include environment-friendly solvents for wide range of chemical processes, electrochemistry, solar cell, and even liquid mirrors that can be set up and used in the Moon. Figures below show some of the current commercial products using IL. The left, middle and right figures show the chemical reactor, solar cell url), and metal oxides dissolved in IL (url), respectably. It can be designed for specific purpose by selecting the cation and the anion.
[1] T. Welton, Chem. Rev. 99, 2071 (1999) url
Bulk ionic liquid
For designing ILs for commercial applications, the fundamental physical properties should be first characterized. However, many physical properties of ILs are not well known yet. For example, ILs are in liquid phase at room temperature (T < 100 oC) unlikely to the usual ionic salts such as NaCl or MgCl2. There has been much effort to understand low melting temperature of ILs in spite of the strong ionic interaction between cations and anions.
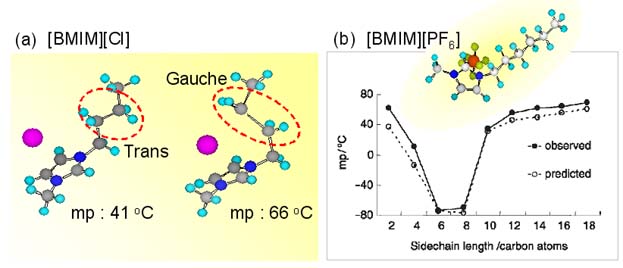
Left below figure represents the relation between the melting temperature and cation structure which was reported by Hamaguchi and coworkers at 2003 [2]. And right below figure represents the relation between the melting temperature and alkyl chain length of the cation which was reported by Seddon and coworkers at 2007 [3]. These results strongly propose us that the alkyl chain attached to the imidazolium ring is one of the most important key in determing the melting temperature. From the point of view, we study the cation structure of ILs.
[2] S. Hayashi, R. Ozawa, H. Hamaguich, Chem. Lett. 32, 498 (2003) url
[3] L. Lopez-Martin, E. Burello, P. N. Davey, R. Seddon and G. Rothenberg, ChemPhysChem 8, 690 (2007) url
cation structure
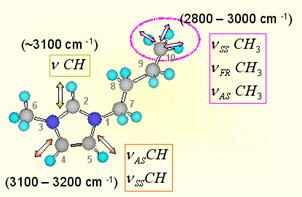
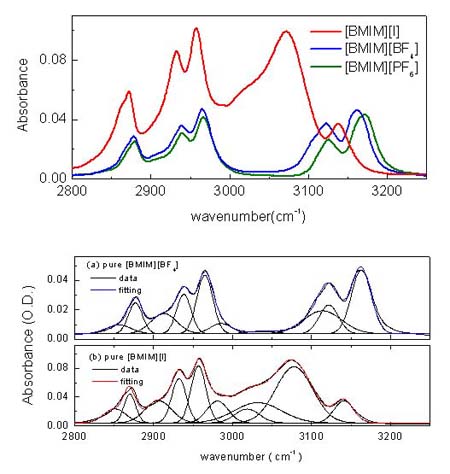
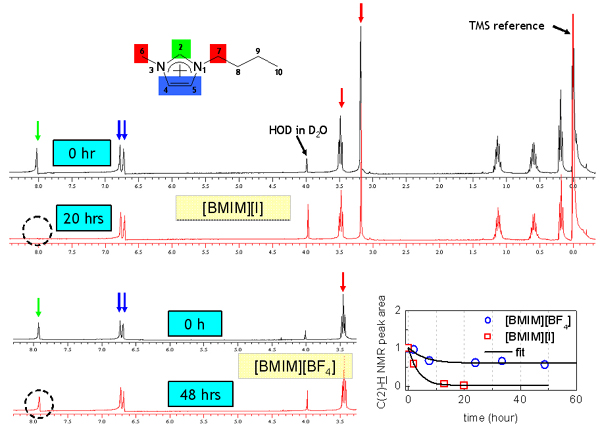
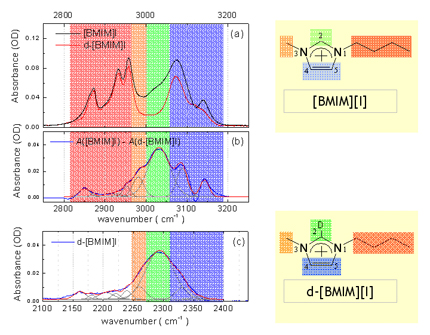
Since [BMIM][BF4] (1-butyl-3-methylimidazolium tetrafluoroborate), [BMIM][PF6] (1-butyl-3-methylimidazolium hexafluorophosphate) and and [BMIM][I] (1-butyl-3-methylimidazolium iodide) are the most popular ILs, so we study them at first. Below figure shows the ATR-IR (attenuated total reflection infrared) spectra for the different ILs. As shown in figure, [BMIM][BF4] (blue line) is very similar with [BMIM][PF6] (olive line), while it is quite different with [BMIM][I] (red line) in the 2800- 3200 cm-1 range which represents the CHx (x = 1, 2, 3) vibration mode peaks of the cation. [4] [5] Second and third rows of the below figure represent the deconvoluted peaks for the [BMIM][BF4] and [BMIM][I] respectively.As shown in the figure, the overall peak intensity of [BMIM][I] is ~2 times larger than the [BMIM][BF4]. Moreover, the CH vibration mode region of the imidazolium ring (3000 - 3200-1) is very different. The symmetric HC(4)-C(5)H stretching mode (vSS HC(4)-C(5)H) peak at ~3163 cm-1 of imidazolium ring is ~2 times larger than the antisymmetric HC(4)-C(5)H stretching mode (vAS HC(4)-C(5)H) peak at ~3126 cm-1 for the [BMIM][BF4] and [BMIM][PF6]. While The symmetric HC(4)-C(5)H stretching mode (vSS HC(4)-C(5)H) peak at ~3141 cm-1 of imidazolium ring is ~5 times smaller than the antisymmetric HC(4)-C(5)H stretching mode (vAS HC(4)-C(5)H) peak at ~3078 cm-1 for the [BMIM][I]. Moreover, the peak position of C(2)-H stretching mode (~3032 cm-1) for the [BMIM][I] is very different. These results propose us that interaction between the imidazolium ring and I- is quite different from interaction between the imidazolium ring and BF4-.
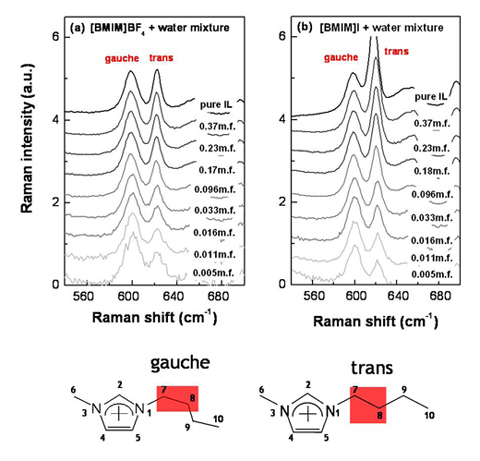
Also, the butyl chain structure of [BMIM][I] is different with [BMIM][BF4]. Below figures represent Raman data for the IL+water mixtures with changing the concentration. At the very dilute cases (~0.005 mole fraction), gauche/trans ratio of [BMIM][I] is very similar with [BMIM][BF4]. However, if the IL concentration is increased, the gauche/trans ratio becomes different for each ILs. For the case of [BMIM][I], the butyl chain have dominantly trans form.
[4] Y. Jeon, J. Sung, C. Seo, H. Lim, H. Cheong, M. Kang, B. Moon, Y. Ouchi, and D. Kim, J. Phys. Chem. B, 112, 4735 (2008) pdf
[5] Y. Jeon, J. Sung, D. Kim, C. Seo, H. Cheong, Y. Ouchi, R. Ozawa, and H. Hamaguchi, J. Phys. Chem. B, 112, 923 (2008) pdf
relative position of anion
NMR (Nuclear Magnetic Resonance)Ionic liquid surface
References:
- ↑ T. Welton, Chem. Rev. 99, 2071 (1999)
- ↑ S. Hayashi, R. Ozawa, H. Hamaguich, Chem. Lett. 32, 498 (2003)
- ↑ L. Lopez-Martin, E. Burello, P. N. Davey, R. Seddon and G. Rothenberg, ChemPhysChem 8, 690 (2007)
- ↑ Y. Jeon, J. Sung, C. Seo, H. Lim, H. Cheong, M. Kang, B. Moon, Y. Ouchi, and D. Kim, J. Phys. Chem. B, 112, 4735 (2008)
- ↑ Y. Jeon, J. Sung, D. Kim, C. Seo, H. Cheong, Y. Ouchi, R. Ozawa, and H. Hamaguchi, J. Phys. Chem. B, 112, 923 (2008)