Lagmuir monolayer
From SMOSwiki
(Created page with "==Langmuir monolayers and pressure/area isotherm== Langmuir monolayer consists of an insoluble molecules floating on aqueous solution and has a thickness of single monolayer. Mo…")
Latest revision as of 13:37, 30 December 2010
Langmuir monolayers and pressure/area isotherm
Langmuir monolayer consists of an insoluble molecules floating on aqueous solution and has a thickness of single monolayer. Molecules consisting Langmuir monolayer are usually ‘amphiphilic’ (means that having both hydrophobic and hydrophilic parts); a common example is fatty acid. Surface tension of water is 73mN/m at room temperature, and repulsion interaction between hydrophilic head group makes the surface tension lower. So, by measuring the surface pressure of Langmuir monolayer versus occupying area of one molecule (surface pressure/area isotherms), thermodynamic phase and the structure of the monolayer can be studied. [1]
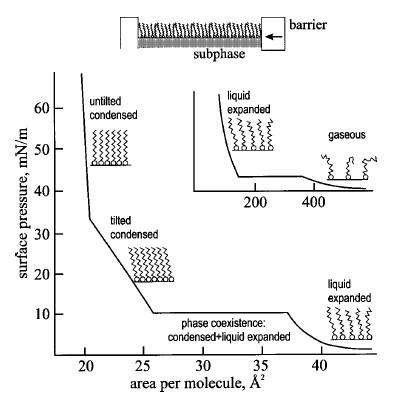
As shown in Fig 1, the region of constant slope in the isotherm indicates the homogeneous phase of the monolayer, while the change in the slope would mean phase transition at that pressure. Detailed monolayer structure can be investigated further by other techniques including X-ray and neutron diffraction [2], ellipsometry, and nonlinear optical spectroscopy.
Fig. 1.The schematic diagram of LB trough and generalized isotherm curve of langmuir monolayer.
Charge inversion phenomenon on the Langmuir monolayers
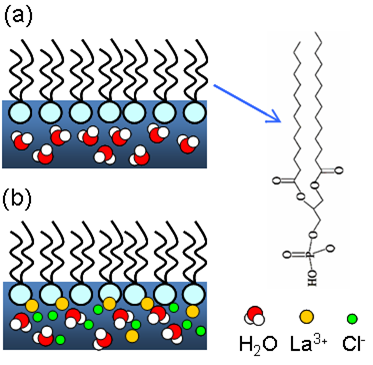
In the electrolyte, one of the interesting phenomena due to electrostatic interaction in soft matter is charge inversion, where charges at an interface attract multivalent counterions in excess of their own normal charge density, causing the sign-change of net surface charges [3]. For example, in the presence of positive multivalent ions (e.g., polycations), the DNA double helix acquires a net positive charge and drifts as a positive particle in an electric field. Analogies of charge inversion in other fields of physics are abundant [4].
Fig. 2. Schematic structure of the interfacial water molecules; (a) before, (b) after charge inversion. Shown at right is the chemical structure of DMPA.
Multilayer molecular films studied by imaging ellipsometry
Arachidic acid (CH3(CH2)18COOH) is a member of the fatty acids composed of carboxyl head group and long alkyl chain tail group. As these molecules easily form a neat monomolecular layer on water surface, the Langmuir monolayer of fatty acid has been studied using various experimental techniques including isotherm, X-ray reflectivity and diffraction, Brewster angle microscopy, Ellipsometry and nonlinear optical spectroscopy[5][6][7][8][9][10][11][12]. Thus structure and conformation of this system for different thermodynamic phases of a monolayer are well understood.
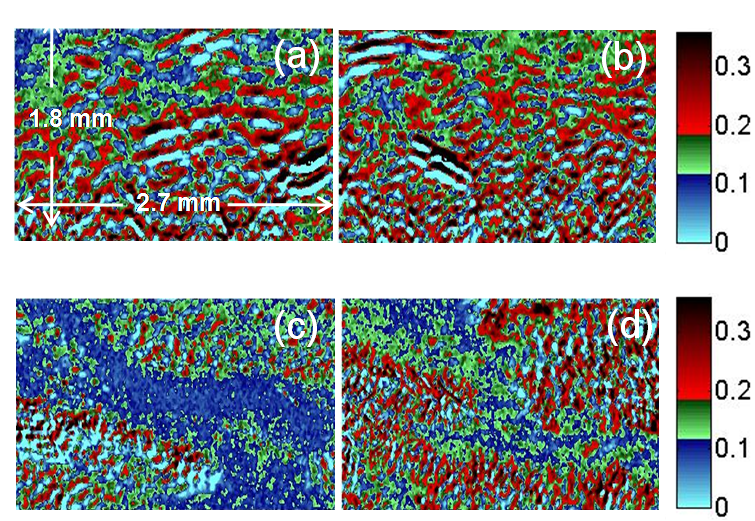
As Barriers of the langmuir monolayer push surface to reduce the molecular area, the process of fatty acid film changing from monolayer into multilayer was imaged in situ. As expected,the surface after collapse was found to be very inhomogeneous with coexsisting regions of bare water surface, monolayer and multilayer films. The thickness of the dominant portion of the collapsed domains correspond to trilayer and bilayer when they are prepared on water and on CaCl2 solution , respectively, supporting the earlier propositon that the thickness of the collapsed region depends sensitively on the existence of salt and water subphase.
Fig. 3 Δ mapping images after collapse ; (a) and (b) collapsed monolayer on pure water, (c) and (f) collapsed monolayer on 1mM CaCl2 solution, area per molecule; (a) 12.5 Å2, (b) 9.5 Å2, (c) 12.3 Å2, and (d) 9.5 Å2.
In usual ellipsometry, two angle component(psi,delta) are determined from ratio between reflected s- and p- polarized light. It has been known that for layers appreciably thinner than the wavelength of probing light the delta value is changed sensitively with thickness of layer while psi isn't.[13] since film thickness in current study is very small with respect to wavelength of light which is used in ellipsometry, IE images depend on delta value. From converting delta value, film thickness can be known.
References:
- ↑ Gareth Roberts, Plenum press. New York and London, Langmuir-Blodgett Films (1990)
- ↑ VM Kaganer, H Möhwald, P Dutta, Reviews of Modern Physics.71, 779-819 (1999)url
- ↑ See, for example, G. Caracciolo, D. Pozzi, R. Caminiti, and H. Amenitsch, Chem. Phys. Lett. 429, 250 (2006).ref3
- ↑ A. Y. Grosberg, T. T. Nguyen, and B. I. Shklovskii, Rev. Mod. Phys. 74, 2 (2002).ref4
- ↑ K. Kjaer, J. Als-Nielsen, C. A. Helm, P. Tippman-Krayer, and H. Möhwald, J. Phys. Chem. 93, 8 (1989).
- ↑ Ronald D. Newman, Colloid Interface Sci. 56, 3 (1976).
- ↑ Dirk Hönig and Dietmar Möbius, J. Phys. Chem. 95, 12 (1991).
- ↑ H. Knobloch, F. Peñacorada, L. Brehmer, Thin Solid Films 295, 210-213 (1997)ref8
- ↑ M. L. Kurnaz and D. K. Schwartz, Phys. Rev. E. 56, 3 (1997).ref9
- ↑ D. Ducharme, A. Tessier, and S. C. Russev, Langmuir 17, 24 (2001).
- ↑ D. Vaknin, W. Bu, S. K. Satija, and A. Travesset, Langmuir 23, 4 (2007).
- ↑ P. B. Miranda, Q. Du, Y. R. Shen, Chem. Phys. Lett. 286, 1-8 (1998).ref12
- ↑ Howland M.C., Szmodius A.W., Sanii B.,Parikh A.N., Biophysics.J.2007,92,1306)ref13